A pain-free solution to vaccines
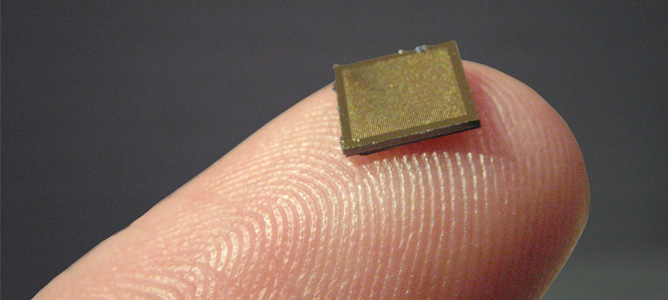
A NanopatchTM placed on a finger to demonstrate its size and application
October 2013
Vaccinations, though vital in preventing the spread of disease in the modern world, have several major drawbacks which limit their effectiveness and uptake in developing countries.
Professor Mark Kendall from the Australian Institute of Bioengineering and Nanotechnology, has spent the last ten years developing the NanopatchTM - a needle free solution to many of the problems facing vaccine use. A tiny square of microneedles coated in vaccine, the Nanopatch is simply placed on the skin and the vaccine dispersed into the immune system just below the skin.
Vaxxas Pty. Ltd., which was established to develop the NanopatchTM, engaged with MCN to increase the outputs of their trial patches. By providing Vaxxas with access to state-of-the-art development equipment and expertise, MCN has accelerated and improved the production of the NanopatchTM.
The NanopatchTM is manufactured using mono-crystalline silicon wafers which are subjected to deep-reactive ion etching (DRIE), the same proccess that is commonly used to fabricate components of smart phones and other microtechnology devices.
MCN has helped to fine tune the NanopatchTM production process so that the layers deposited on these wafers achieves better uniformity, increasing the output from each wafer. Furthermore, MCN has allowed Vaxxas to move from four inch wafers to six inch wafers, once again increasing the yield. MCN also hosts two DRIE systems which enables them to double their final outputs by processing wafers in parallel.
Eliminating the pain of traditional injections is just one small advantage of the NanopatchTM. As the vaccine is dry coated onto the patch, the need for refrigeration is eliminated. This vastly increases its stability and reduces cost barriers for transporting vaccines to where they are needed most in third world countries. The NanopatchTM has the capacity to transform the world’s disease landscape, and to stamp out illnesses in third world countries that have long been preventable in the developed world.
NB: The material for this case study has been provided by Vaxxas Pty. Ltd. Although care has been taken to ensure the accuracy and completeness of the information that is provided, the MCN assumes no responsibility in relation to the interpretation or use of this information.
More information can be found at www.vaxxas.com.au


