Next generation pathogen detection with lab-on-a-chip platforms
Taking a sample could be as simple as a fingerprick of blood, as is common in blood sugar monitoring.

The metal microdiscs sputtered on a glass slide at MCN. The posts are 5 µm in diameter and 300 nm high.
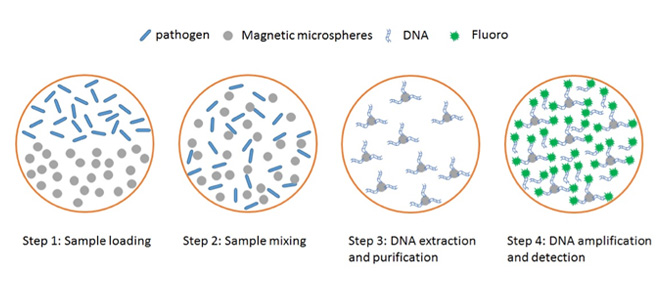
The schematic drawing of the pathogen detection: DNA extraction, purification, amplification and detection.
March 2015
Detection of pathogens is of critical importance for disease diagnosis, food safety control, water quality monitoring and homeland security. Traditional detection methods, such as colony counting following cell culture, polymerase chain reaction (PCR) and enzyme immunoassay, have been extensively employed to pathogen detection. But they require special laboratories, trained professionals and hours, if not days, to achieve the required analysis.
The portable lab on a chip platform developed in this project enables the detection of multiple pathogens within 30 minutes, with only a tiny sample, such as a drop of blood or saliva. The detection process is as easy as blood sugar monitoring used daily by diabetics: drop the sample on a disposable microchip, measuring just 5cm x 7cm x 0.4 cm, insert the chip into the reader and read the results after half an hour. It is fully automatic and no longer relies on sophisticated equipment and trained professionals. Furthermore, it not only greatly reduces the complexity and detection time but dramatically increases detection sensitivity. It holds great potential to benefit the medical clinics, food industries, as well as departments of environmental monitoring and homeland security.
Led by Dr. Yonggang Zhu, CSIRO researchers from multiple disciplines have been collaborating with industry partners to develop a lab-on-a-chip platform with three key capabilities including automatic liquid handling, sample processing and individual bead trapping and imaging. The liquid handling system is applied to load the samples (such as blood, saliva, urine, etc.) and reagents are placed into a microwell for further processing. Then magnetic microbeads and an electromagnetic/acoustic system are applied to mix and capture the analyte. Finally, the microbeads coupled with analyte are individually captured and imaged by fluorescence microscopy or Surface Enhanced Raman Spectroscopy (SERS).
The core component of this platform is the microfluidic chip with microchannels, a microwell and metal microstructures for the sample processing and detection. It is fabricated using the photolithography facilities and sputtering tools at MCN.
Proof of concept studies have shown successful detection of DNA from E. Coli (bacteria) using this lab on a chip platform. DNA was lysed from whole E. Coli cells and purified on the chip within 15 minutes. Then the small number of DNA molecules were amplified in situ for a further 15 minutes. Following the amplification, concentrated DNA molecules weres able to be detected using fluorescence microscopy.
Future work will focus on the optimisation of the platform in terms of hardware, detection methods and software to further shorten the detection time, reduce the detection limit (therefore detecting fewer pathogens in a given volume) and increase its ability in detecting multiple pathogens.
Microchips to mimic living cells
A microchip that can generate microdroplets for protein and nanoparticle synthesis.
July 2013
Together with MCN, Technology Fellow Yonggang Zhu and his team from CSIRO have successfully developed a microchip that mimics a living cell by housing millions of tiny water droplets which synthesise enzyme molecules. This is a critical step towards fast-tracking low cost protein development.
They have successfully developed a microchip that can produce millions of tiny water droplets inside itself. These droplets contain necessary materials like those in a living cell, while enzyme molecules are synthesised in each droplet. The unprecedented speed of synthesis can potentially solve some of the bottle-neck problems in protein evolution.
The team has studied the physics of fluid flow at microchannels and designed a microchip that can flow two immiscible flows i.e. water and oil, for generating monodispersed water-in-oil microdroplets. The microchip was fabricated at MCN using polymeric materials instead of silicon. The plastic microchip has the function of sequentially generating microdroplets for compartmentalising biological reagents, performing in-drop in vitro transcription and translation reactions, fusing droplets for on-chip enzyme assays and detecting in-droplet biochemical reaction products.
The microchip format allows the generation of millions of enzyme assay droplets per day, which is an unprecedentedly high throughput none of the currently commercially available equipment can match. The progress Dr. Zhu’s team has made in microchip-based enzyme synthesis and screening is significant. Since tiny amounts of sample solution is required and a high throughput can be achieved, this could potentially allow researchers to screen and select libraries of massive numbers (from millions to trillions) of candidate gene/protein variants, from either natural sources or synthetic ‘in vitro evolution’ experiments, a critical step to fast track the protein development with low cost.
The technology could potentially allow biologists to develop new proteins (e.g. enzymes) within days instead of years and with a cost of hundreds of dollars instead of millions and billions of dollars. This will benefit many areas in biotechnology, medicine and environment.
You can read more about this work in Enzyme synthesis and activity assay in microfluidic droplets on a chip, published in Engineering in Life Sciences.
Protecting the nations’ water supply

Nanostructural features present on a microfluidic wafer.
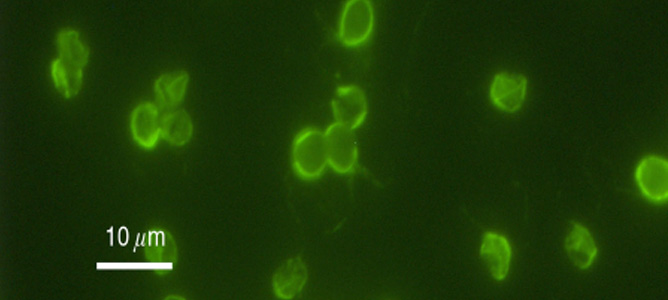
Cryptosporidium Parvum Bacterium
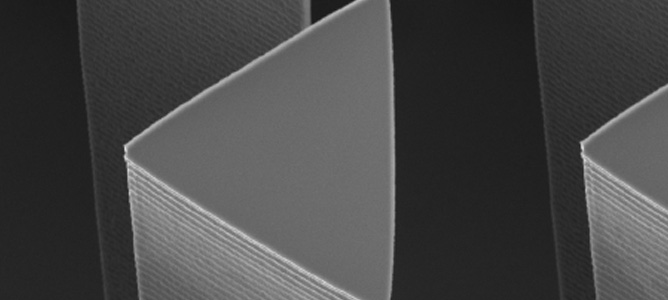
Close up of nanostructural features present on a microfluidic wafer.
March 2012
Microfluidic sensing platforms capable of sorting and filtering nanoparticles within aquatic sensor systems may hold the key for future pathogen detection within community water supplies.
Using a fluid separation element fabricated within MCN’s state-of-art photolithography and clean room facility, this device harnesses the process of surface acoustic waves to actively filter harmful disease-causing pathogens. Such pathogens include the Cryptosporidium Parvum Bacterium, responsible for the 1998 Sydney water crisis.
Given the expertise and unique array of equipment available with the MCN, the facility has allowed the technology to be completely transposed from design to development within a single place. Hosted by MCN, this work forms part of a cluster collaboration between CSIRO and Australian universities to create an integrated device which may one day filter our nation’s water supply.
Synthesising high quality enzymes
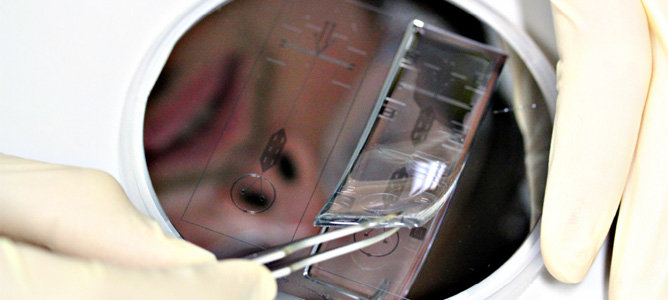
Microfluidic device designs for the production of high quality enzymes
March 2012
Enzymes, organic catalysts capable of facilitating physicochemical and biological reactions, have captured the attention of industry and the scientific community alike. In nature, enzymes are used to catalyse and break down proteins. New methods of synthetic production have vastly broadened the applications of such enzymes to include food processing, textile and paper production, biological detergents and farming, medical and therapeutic processes, fuel production and sustainability practices.
According to Florian Lapierre from CSIRO, one of the major challenges of synthetic enzyme production lies not in the overall yield size, but achieving a product that is of consistently high quality. Using the soft lithography capabilities within the MCN’s cleanroom facility, a micro droplet sorting device has been fabricated and is currently undergoing extensive testing. The prototype is capable of generating amino acid emulsions, synthesising proteins via electrically induced coalescence, detection and subsequent sorting of high quality enzymes. The result is a purified sample, which can then be used for catalytic processes or stored for later use.
Acoustic nanofluidics

Fluid reservoirs are connected with an output well via 100, 50, and 20nm wide channels in lithium niobate. Upon filling the reservoirs and starting acoustic radiation in the substrate, the channels rapidly fill the output well at speeds many orders beyond physically predicted rates. This entire structure would fit in this full stop.
October 2011
Simulated molecular dynamics at the nanoscale show tremendous potential for improved manipulation of particles and molucules in fluids. In his fellowship project, Professor James Friend is examining the phenomena of rapid fluid flow in nanochannels induced by surface acoustic waves. Very high frequency sound waves (10–1000 MHz) applied in microchannels enables pumping, mixing, particle separation and other phenomena useful for medical diagnostics and chemical detection devices.
At the nanoscale, molecular dynamics simulations are showing potential for over five orders of magnitude improvement on any current method known for manipulating fluids and nanoparticles and molecules within.
At MCN, the focused ion beam and electron beam lithography facilities, the UV lithography and deep reactive ion etch instruments are all crucially important in fabricating the acoustic wave devices and the nanostructures on them. These facilities are especially convenient as they are adjacent to laboratories at the MCN where the devices can be immediately tested on confocal and high-speed fluoroscopy equipment. The devices go from concept to fabrication to testing in a matter of a few days.
This work has the potential to benefit Victorians through potential means of efficient drinking water filtration, drug delivery and implantable medical device technology to treat cancer and autoimmune diseases, as well as acoustophoresis for protein and DNA assays to serve as a tool in microbiochemistry for identifying the causes of disease and illness.
Evolving enzymes on a microchip
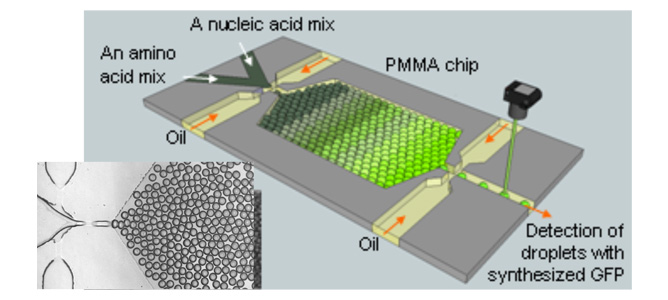
A picture of a microchip developed in CSIRO Microfluidics Lab for synthesis of a green fluorescence protein molecule
October 2011
Dr. Yonggang Zhu’s Fellowship project aims to develop an integrated microfluidic chip that can perform in vitro enzyme synthesis and evolution. This technology could potentially revolutionise nano- and bio-technology development.
The current protein evolution protocol requires screening of ‘libraries’ of massive numbers of candidate gene/enzyme variants, at a cost between millions and billions and take several years to complete. This project proposes to use microfluidic water-in-oil droplets to compartmentalise these libraries. These discrete micro-droplets are ideal bioreactors in which enzymes can be synthesised, screened and selected. The high level of controllability and speed of droplet production in microchips (~103 drops per second) allows for unprecedentedly fast and low-cost screening of massive libraries, with a potential 106-fold reduction in time and cost in comparison with existing technologies.
MCN’s state-of-the-art lithographical tools, characterisation tools and biological labs will be fully utilised for the project.
This work could potentially lead to a new way of developing novel biomolecules. Such a development would benefit many industry sectors such as biotechnology, chemical manufacturing, in vitro cell-free protein evolution, biomaterials, sensing and drug development.


