Metal nanoparticles lead the way towards solar water decontamination
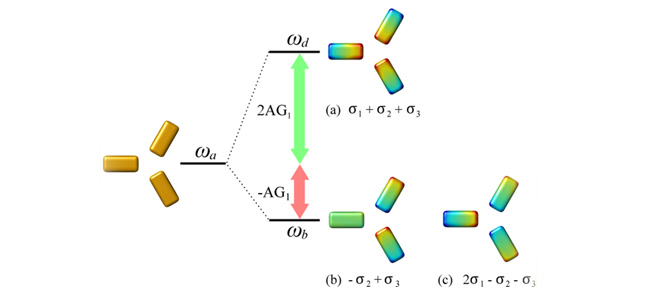
Three lowest-order modes of an interacting triangle of particles. Mode a) is the highest in energy of the three and is a mode with radial symmetry. Modes b) and c) are degenerate modes which are characterised by having a net dipole moment and perpendicular polarisations.
April 2014
It has long been known that solar energy can be used to create electric energy for heating and lighting. It can also be used however, to drive important chemical transformations such as the production of renewable chemical fuels by extracting hydrogen from water and hydrocarbons from carbon dioxide. Other uses include the sequestration of air pollutants such as greenhouse gases, low-cost water purification for developing countries, and the low carbon-footprint production of fine chemicals such as disinfectants and self–cleaning surfaces. There are countless other as yet unforeseen applications of solar to chemical energy transformations.
Existing approaches for harnessing solar energy for these applications are relatively inefficient and therefore not widespread. In order to improve the efficiency of solar-to-chemical energy conversion processes, it is important to maximise the light collection efficiency.
In this project, researchers from CSIRO led by MCN Technology Fellow Dr. Daniel Gomez, are creating novel metal nanoparticles capable of efficient light collection and focusing, in order to enhance the efficiency of light-driven chemical reactions. Utilising the Electron Beam Lithography (EBL) system, thin film deposition and characterisation tools available at MCN, the team have created networks of precisely controlled metal nanoparticles with precision down to 10nm.
Preliminary results indicate that certain nanoparticle arrangements can lead to an enhancement of light-driven decomposition of water pollutants by at least eight times the current rate of decomposition. They have achieved this by creating arrays of nanoparticles using aluminium, an abundant and inexpensive metal, which offers great potential for future deployment in large-scale applications.
The team are currently optimising the shapes and geometrical arrangements of the nanoparticles to achieve optimal rates of photochemical rates. Through this optimisation process they are addressing key fundamental questions, which will provide guidance in the development of processes for creating complex hydrocarbons from carbon dioxide.
You can read more about this project in The Dark Side of Plasmonics, published in Nano Letters.


